Food Coloring Law
In 1906, Congress passed the Food and Drugs Act, which prohibited the use of poisonous or deleterious colors in confectionery and the coloring or staining of food to conceal damage or inferiority. Food ingredients as authorized by a definitions and standard of identity prescribed by regulations pursuant to section 401 of the act are color additives, where the ingredients are specifically designated in the definitions and standards of identity as permitted for use for coloring purposes. Both state and federal government authorities have recently turned their attention toward artificial coloring in foods.

The Food and Drug Administration (FDA) announced a series of measures in. Navigate food coloring regulations and standards with HunterLab. Explore insights into compliance and quality control for the food industry.

The Surprising History of Artificial Colors in Food - ID Times
The legal definition can be found in Section 201(t) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) and provides exclusions as well. Color additives for use in food, drugs, and cosmetics require premarket approval. FDA has regulatory oversight for color additives used in foods, drugs, cosmetics, and medical devices.

Bright red candies or neon orange drinks may look appealing, but their ingredients are raising questions about public health and safety, and legislatures are taking notice, especially when it comes to children. As of March, lawmakers in 20 states have introduced nearly 40 bills aimed at regulating or banning dyes and other food additives. A study published in the June 2025 issue of the Journal.

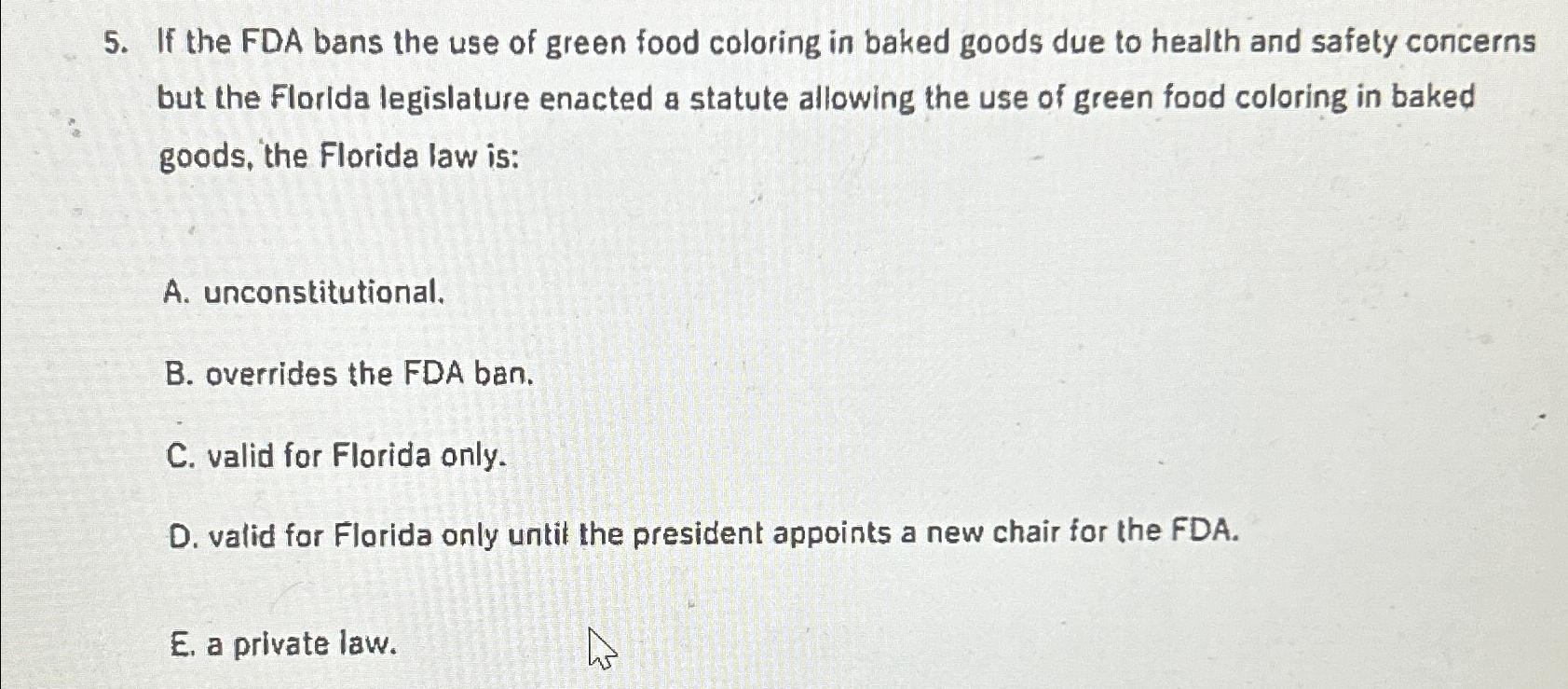
Solved If the FDA bans the use of green food coloring in | Chegg.com
A color additive, 21 CFR 70.3(f), is any substance that imparts color to a food, drug, cosmetic, or to the human body. Color additives can be naturally derived or synthetic substances. The purpose of this article is to explain how color additives are regulated in the United States, specifically the laws and regulations that apply to color additives, how the U.S.
Food and Drug Administration (FDA) evaluates their safety and how FDA ensures their compliance with these laws and regulations. A Regulatory History and Framework U.S. regulation of color additives can trace its.

Food Coloring Regulations and Standards | HunterLab
In the labeling of foods, most certification-exempt color additives may be declared as "artificial color" or "artificial coloring" (21 CFR 101.22 (k) (2)), while certified color additives must be. In a long-overdue but pivotal move, U.S. Health Secretary Robert F.

Kennedy Jr. has announced that eight synthetic food dyes will be removed from the U.S. food and pharmaceutical supply by the end of 2026.

Found in everything from candies and sodas to medications and children's vitamins, these dyes have long functioned as marketing tools.