What Happens When You Add Food Coloring To Water
When food coloring is added to water, a series of fascinating events unfold, transforming the colorless liquid into a vibrant, colored solution. This phenomenon is not just visually appealing but also offers insights into the fundamental principles of chemistry and physics. In this article, we will delve into the world of food coloring and water, exploring the chemical reactions, physical.

Mixing food coloring and water is a simple yet fascinating experiment that captivates both children and adults alike. The vibrant hues that emerge when these two substances combine not only create a captivating visual effect but also provide valuable insights into the principles of chemistry and physics. In this comprehensive piece, we will explore the science behind food coloring, the.

What Happens When You Add a Drop of Food Coloring to Cold Water? | Sciencing
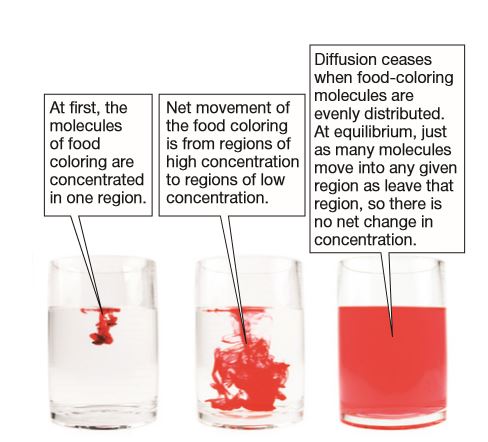
Food coloring illustrates diffusion in water. Diffusion is the mixing of molecules due to their random motion, whether in a liquid or a gas. Because molecules in cold water have less kinetic energy than in warm water, the diffusion process is much slower than in warm water.

But the food coloring can also show movement that isn't random, such as agitation of the water by convection. So, the next time you add a drop of food coloring to water, take a moment to appreciate the science behind the spectacle. It's a reminder that even the simplest things can reveal profound insights into the nature of the universe.

What Happens When You Add a Drop of Food Coloring to Cold Water? | Sciencing
Water, the essence of life, plays a vital role in countless chemical reactions, including the fascinating behavior of food coloring. When food coloring is introduced to water, an interplay of diffusion, solubility, and concentration gradients creates visual and scientific phenomena. This article will explore the intricacies of what happens to food coloring when it meets water, how different.

When food coloring is added to water, it spreads throughout the water in a process called diffusion. The coloring continues to spread until all parts of the water contain an equal concentration of the dye. The food coloring you add to the water is pushed around by the water molecules.

Food Coloring Water Experiment at Samantha Atherton blog
Since the molecules in warm water move around faster, the food coloring spreads out quicker in the warm water than in the cold water. What happens when you add food coloring to the cup of room temperature water? These fast-moving molecules are pushing the molecules of food coloring around as they move, causing the food coloring to spread faster. The process of adding food coloring to water involves the dispersion of the food coloring molecules within the water molecules, resulting in the characteristic color of the water.

This dispersion is a physical change because it does not alter the chemical structure of the food coloring or water. If you were to evaporate the colored water, you would be left with the same food coloring you. Mixing food coloring with water is a common experiment in both kitchens and classrooms.

The vibrant colors that food coloring brings can transform any dish or art project. But have you ever stopped to consider what happens at a molecular level when you mix food coloring with water? Is it merely a physical change, or is it a chemical transformation? This article will explore the science behind.