Chemical Burning Colors
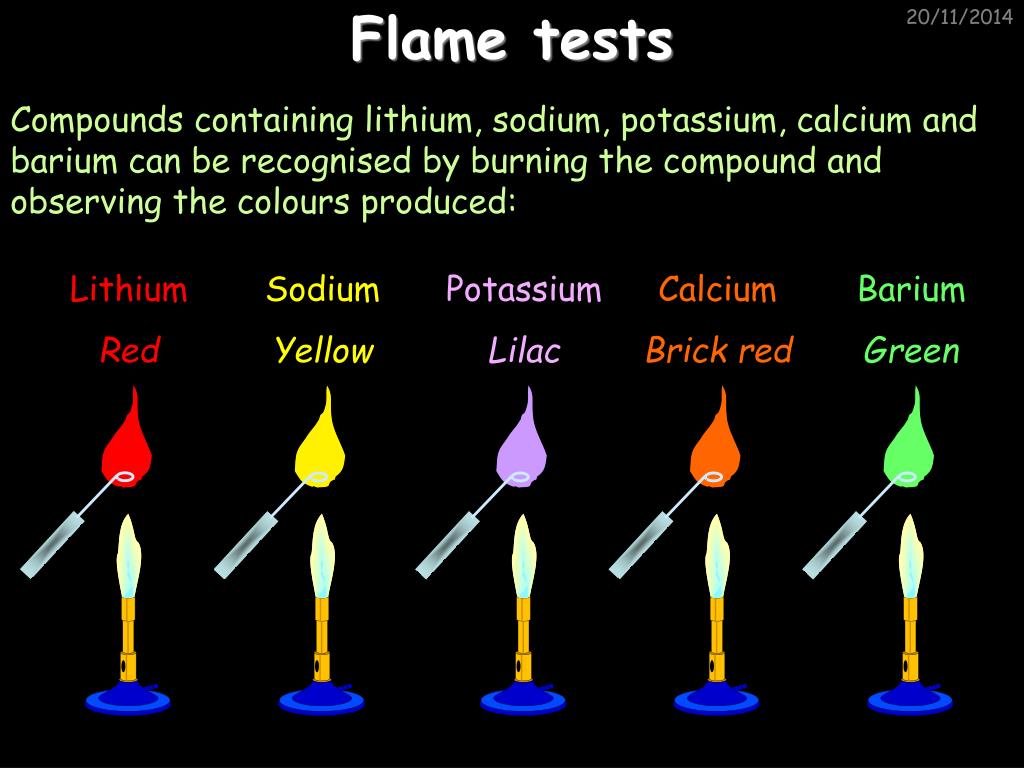
Sodium compounds glow yellow in a flame. A pyrotechnic colorant is a chemical compound which causes a flame to burn with a particular color. These are used to create the colors in pyrotechnic compositions like fireworks and colored fires.

The color. The other way to change the color of th fire is to burn different chemicals. All elements burn at different temperatures and show different colors as they burn.

What Color Does Methane Burn? - Color Box Hà Nội
Magnesium metal, for instance, burns with a whitelight. Don't stare at it though becauseit burns so brightly that it can scorch your eyeballs! Magnesium, potassium and titanium, all elemental metals, are commonly used in fireworks to. Learn how to make colored fire at home in your fireplace or campfire.

See which chemical produce the colors of the rainbow and where to find them. Combustion is the burning of molecules to produce heat and light. Different molecules produce different colored flames.
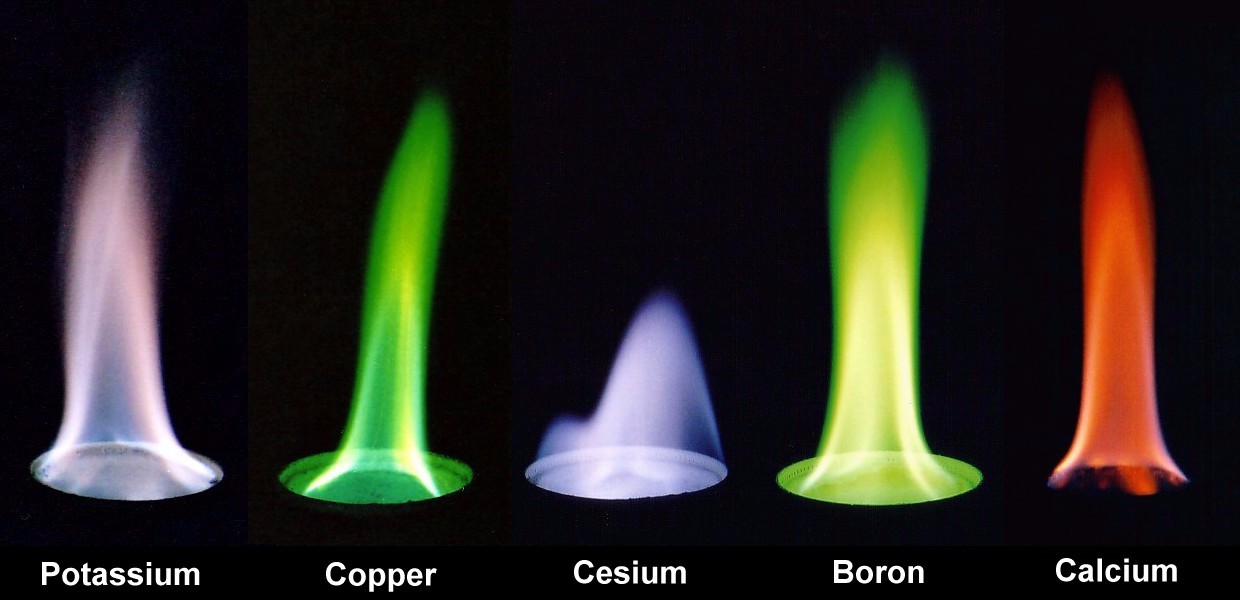
Courtesy of Compound Interest (2014)
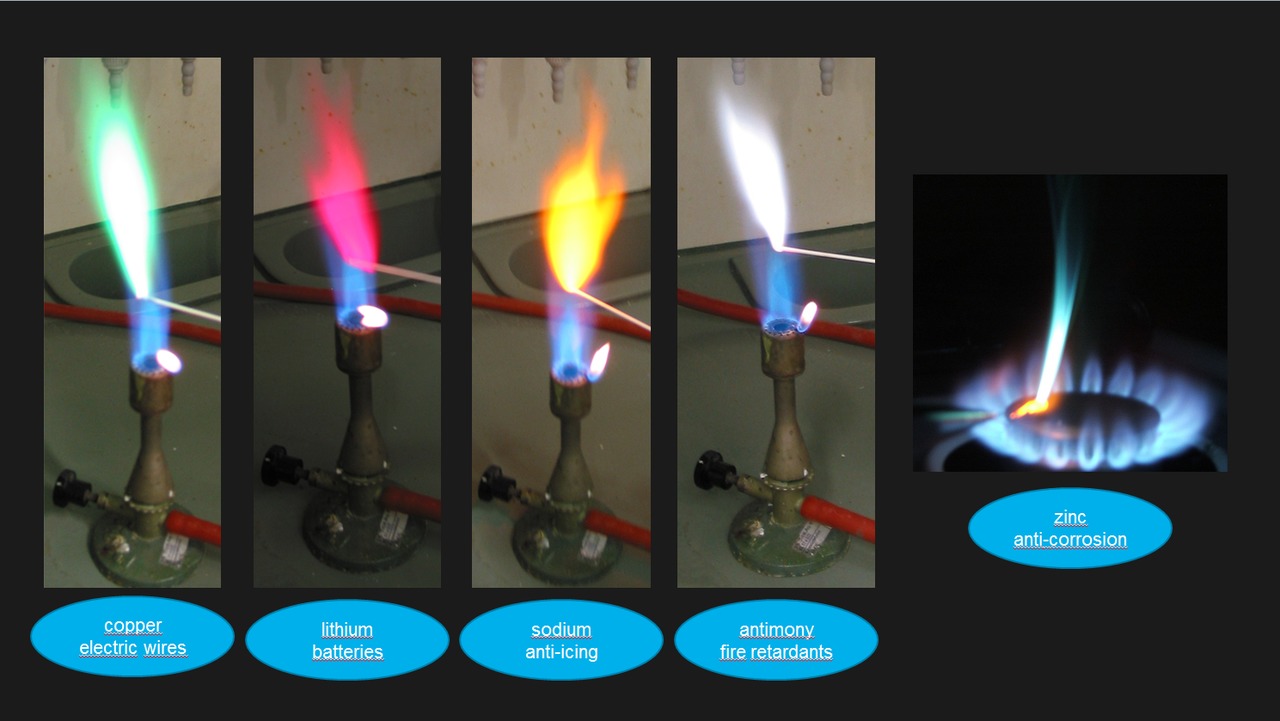
The color of each flame depends on the energy released by the electrons of the atom during de-excitation. In the case of carbon fuels, the color of the flame depends on the amount of oxidation that the carbon molecules undergo. The Role of Elements and Chemicals Beyond temperature, specific chemical elements within burning material can dramatically alter flame color.

When heated, their electrons absorb energy and jump to higher levels. As these excited electrons fall back, they release absorbed energy as light at specific wavelengths, a process called atomic emission. Home > Info & How To > Science Experiments > Creating Flame Colors Creating Flame Colors For a fun and colorful campfire or fireplace display, you can soak pine cones, wood chips or newspaper-rolls in chemical solutions prior to burning.

Combustion chemical reaction Stock Vector Images - Alamy
Whether for personal use or as a club/group project, we indicate what to do and what to use to create these flame displays. Project 1: Campfire or Fireplace. Sodium chloride at left gave the brightest light, much brighter than the blue flame of the burner.
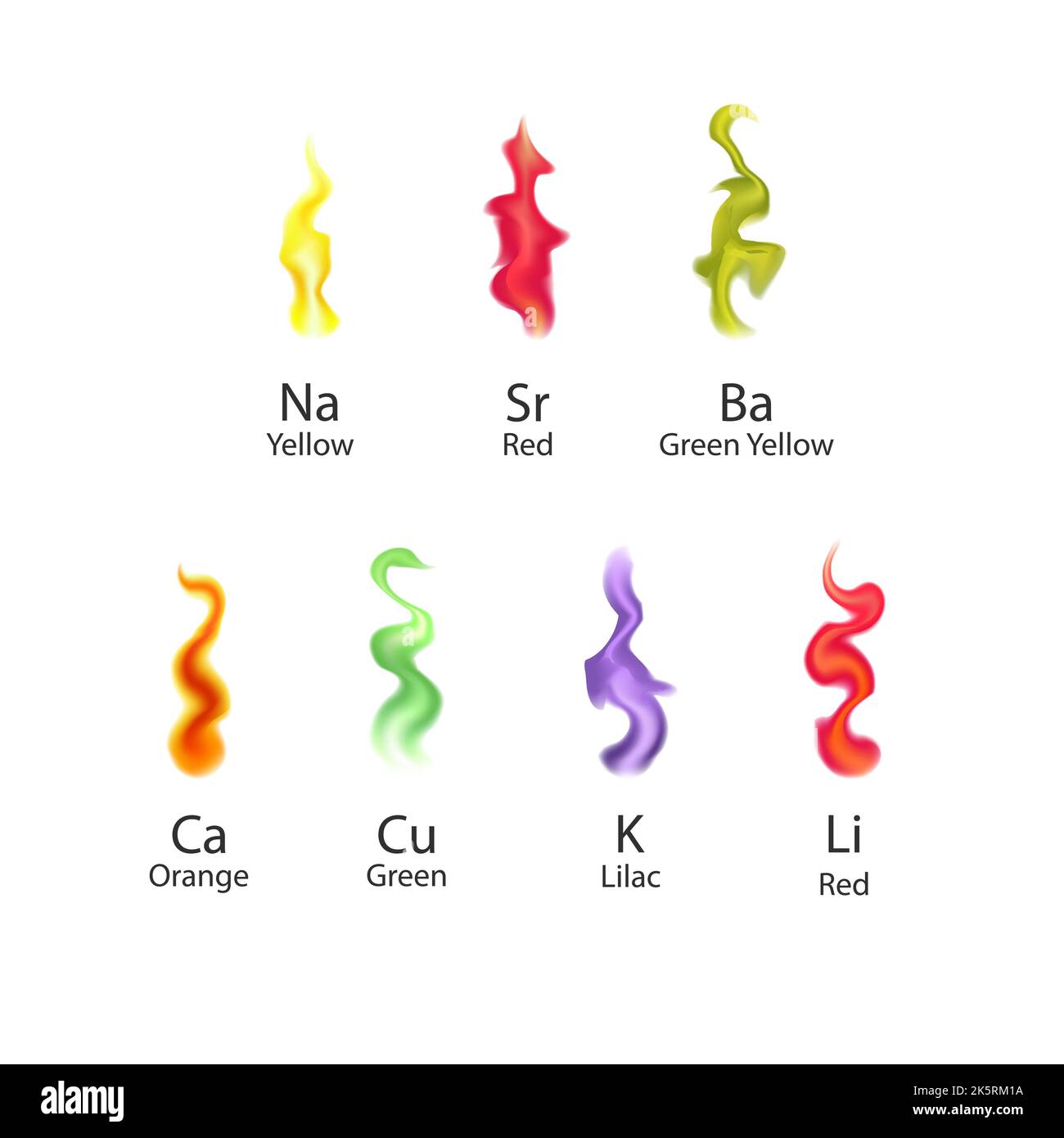
The color is visually the same as other sodium lights, coming mainly from the sodium d-lines. The brilliant red of strontium is the most dramatic of the flame colors. At right above is a sample of barium sulfate (barite) mineral, which gave only a small amount of light which was almost white.
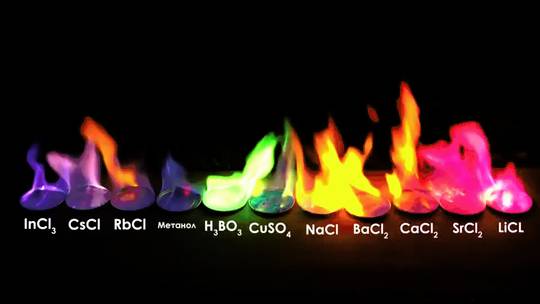
Discover the fascinating world of flame colors and their hidden meanings in our comprehensive guide. Learn how different flame colors, such as blue, green, and purple, indicate chemical compositions and temperatures, making it an essential tool for chemistry, pyrotechnics, and metalworking. Explore the uses and significance of flame tests, color spectroscopy, and flame color charts in various.

This chemical compound produces a blue. Learn how firework colors work, including the characteristic colors of elements and other chemistry that makes color and special effects.