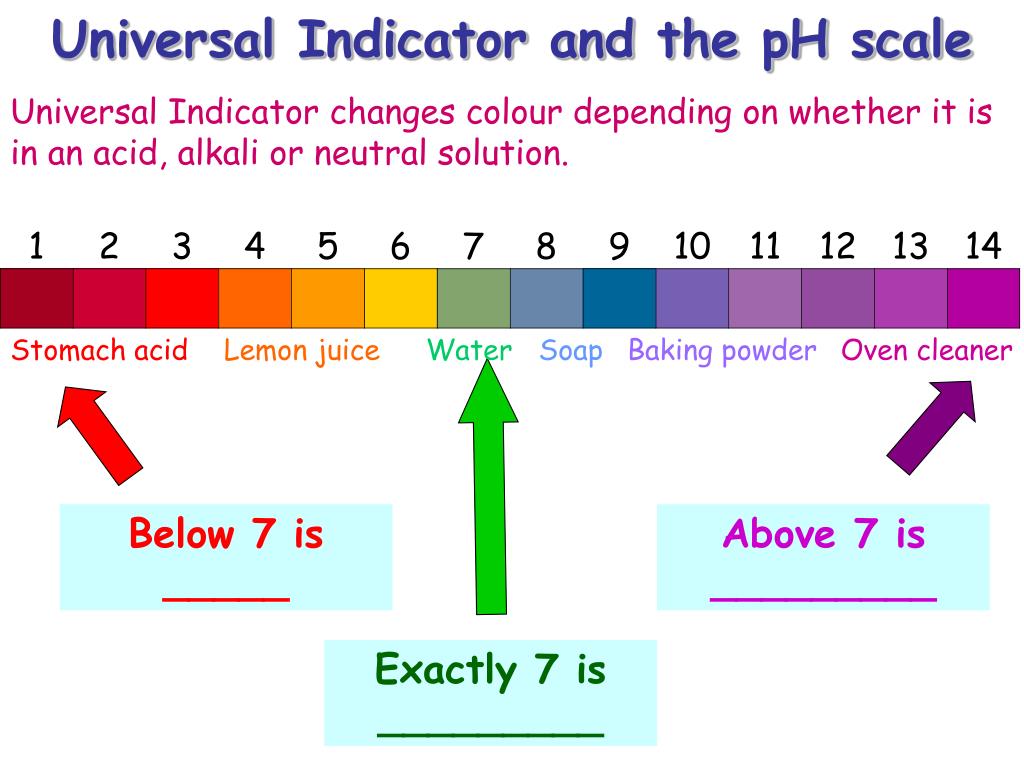
Fruit Juice Colour In Universal Indicator
Download and print a universal indicator color chart. Learn how to make universal indicator solution and interpret pH results. Lemon juice In the lemon juice experiment, the pH paper turns from blue to vivid red, indicating the presence of \ (H_3O^+\) ions: lemon juice is acidic.

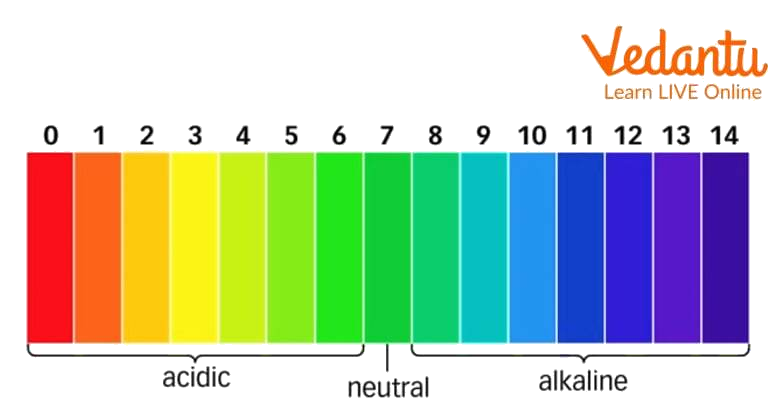
Refer to the table of Universal Indicator Color change (figure 1 in the introduction) for clarification. Find the approx pH of vegetable and fruit juices using a universal indicator and pH paper. Includes color changes for lemon & tomato juices.
Lemon Juice Universal Indicator, HD Png Download - vhv
Requirements Test tubes, measuring cylinder, glass rod, watch glass, vegetable and fruit juices, universal indicator solution and pH paper. Procedure 1. Using pH Paper.
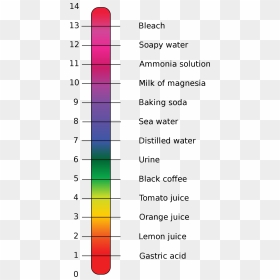
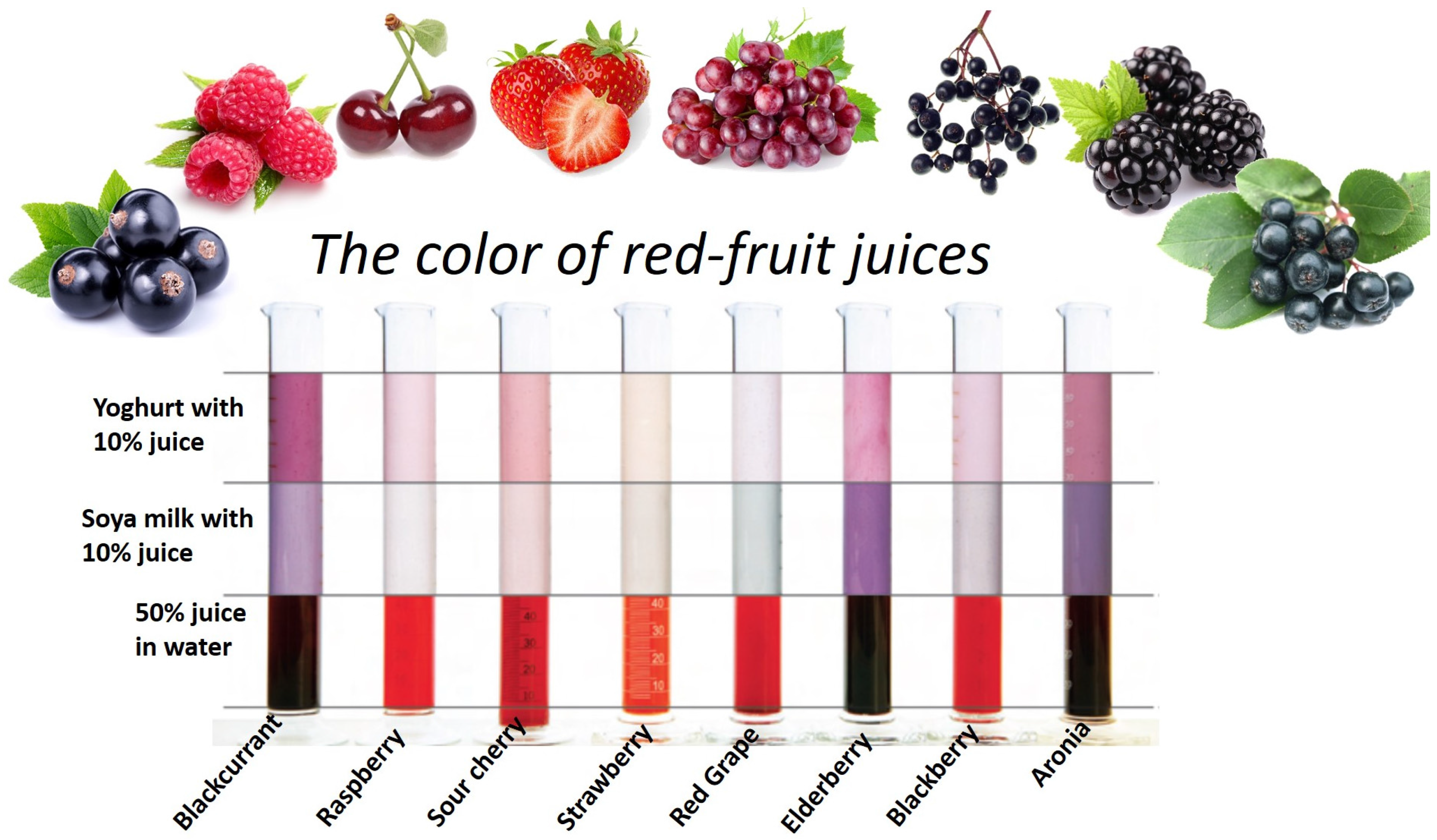
Take some clean and dry test tubes and place various samples of vegetable and fruit juices in each of them. Now put one or two drops of each sample on different strips of pH papers. This is a color change chart for edible acid.

Determination of pH of Vegetable & Fruit Juices for Class 11 Chemistry
Now compare the colour of the pH paper strip to the standard pH chart. Note down the value of the pH of the tested samples. Using Universal Indicator Take a given sample of juices like lemon juice, orange juice, tomato juice, pineapple juice, amla juice and mango juice in a separate test tube.
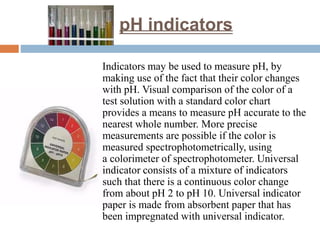
The color of universal indicator in lemon juice will be red. Universal indicator is a mixture of different pH indicators that change color based on the acidity or alkalinity of a solution. The universal indicator contains a set of chemicals that change colour depending on the amount of acid or base substances in the solution (Figure 2.37).
Neutralization Cabbage Juice Demo - ppt download
They work by producing extra protons depending on how acidic or alkaline the solution is, which in turn emit photons with different wavelengths. Figure 2.37. A universal indicator will undergo a variety of color changes in different pH solutions.

Hydrion paper is a convenient way for scientists to be able to exactly measure the pH of a solution. Red cabbage juice is the best known edible pH indicator, but there are many safe fruits, vegetables, and flowers that change color in response to acidity or alkalinity. The plant pigments responsible for the color change are anthocyanins.

Most of these molecules change from red to purple to blue in response to pH.